How to and Educational Videos







Lab Talk Episode 25: Maximizing Sustainability: Exploring Pipette Tip Recycling in the Lab
21:23
Watch as we explore sustainable practices in the laboratory by discussing the benefits and best practices associated with pipette tip recycling, aiming to reduce environmental impact and promote responsible laboratory waste management.
Utility of NGS in Lymphoid Malignancies and the Liverpool Lymphoid Network Panel experience
17:37
Learn how the Ion AmpliSeq Liverpool Lymphoid Network Panel can provide fast and comprehensive assessment of multiple lymphoid disorders in a single test.
Serum Smart: Where in the world is Grand Island, NY?
2:47
In this episode of Serum Smart, learn about Grand Island, NY and it's important history, and impact to cell culture research.
Avoid dish disasters with Nunc EasYDish cell culture dishes
1:00
Thermo Scientific Nunc EasYDish round cell culture dishes are designed to improve handling, stacking, and transporting of cell cultures in a lab.
LysoLight Deep Red antibody internalization
0:22
LysoLight Deep Red provides a powerful tool for visualizing the lysosomal delivery and catabolic degradation of antibodies and proteins.
How to optimize your parameters on the Neon NxT system
6:21
This video shows you the key steps to optimize your parameters on the Neon NxT Electroporation System. By understanding the importance of factors like pulse voltage, pulse width, pulse number, cell density, cell health,
Care & maintenance: Thermo Scientific™ SpeedVac™ vacuum concentrators
4:36
Learn how to perform routine care and maintenance for your Thermo Scientific SpeedVac Vacuum concentrator, so you can ensure years of functionality.
Centrifuge Room Virtual Experience
1:10
Tour the Thermo Scientific centrifuge room virtual experience.
ARL iSpark Plus OES Metal Analyzer – Taking speed and stability to the next level
3:07
The ARL iSpark Plus metal analyzer, the next generation of the trusted standard in spark optical emission spectrometry, streamlines your laboratory workflow by taking speed and stability to the next level.
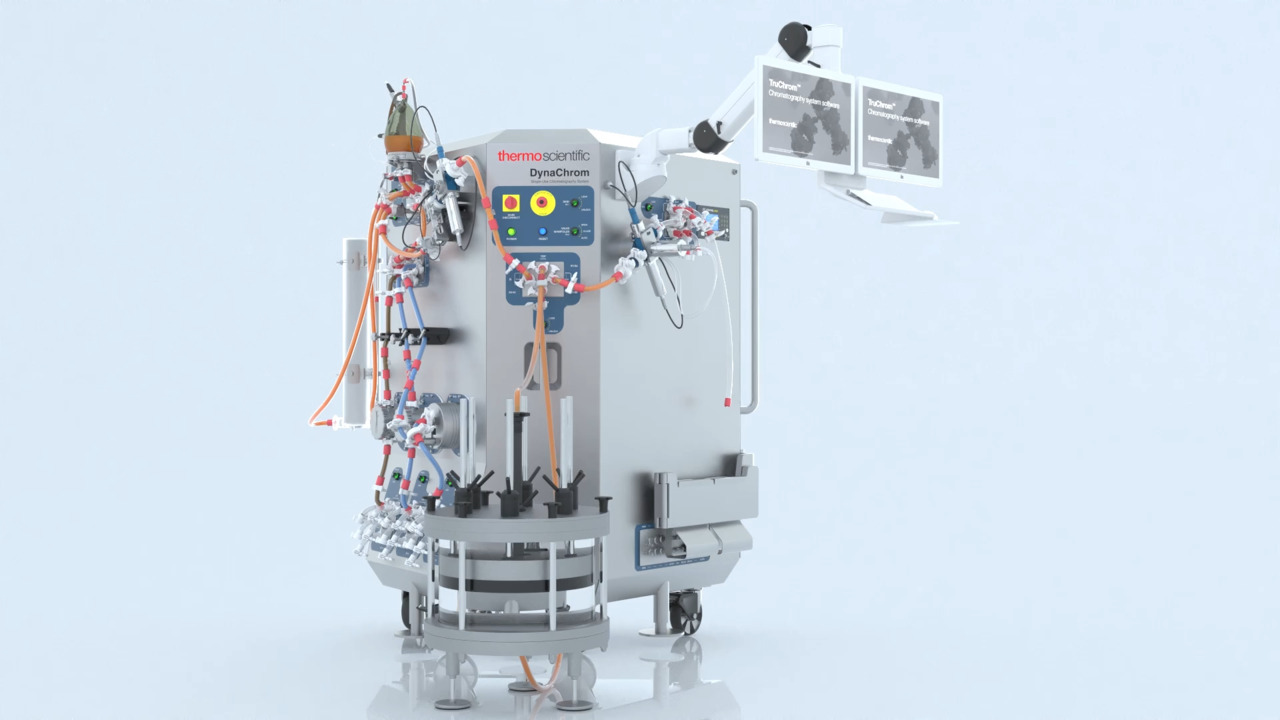
The Thermo Scientific DynaChrom Single-Use Chromatography System
2:41
DynaChrom is a configurable system that alleviates operational burdens, streamlines workflows, and provides the tools necessary for a successful and hassle-free chromatography experience tailored to your unique requirements.
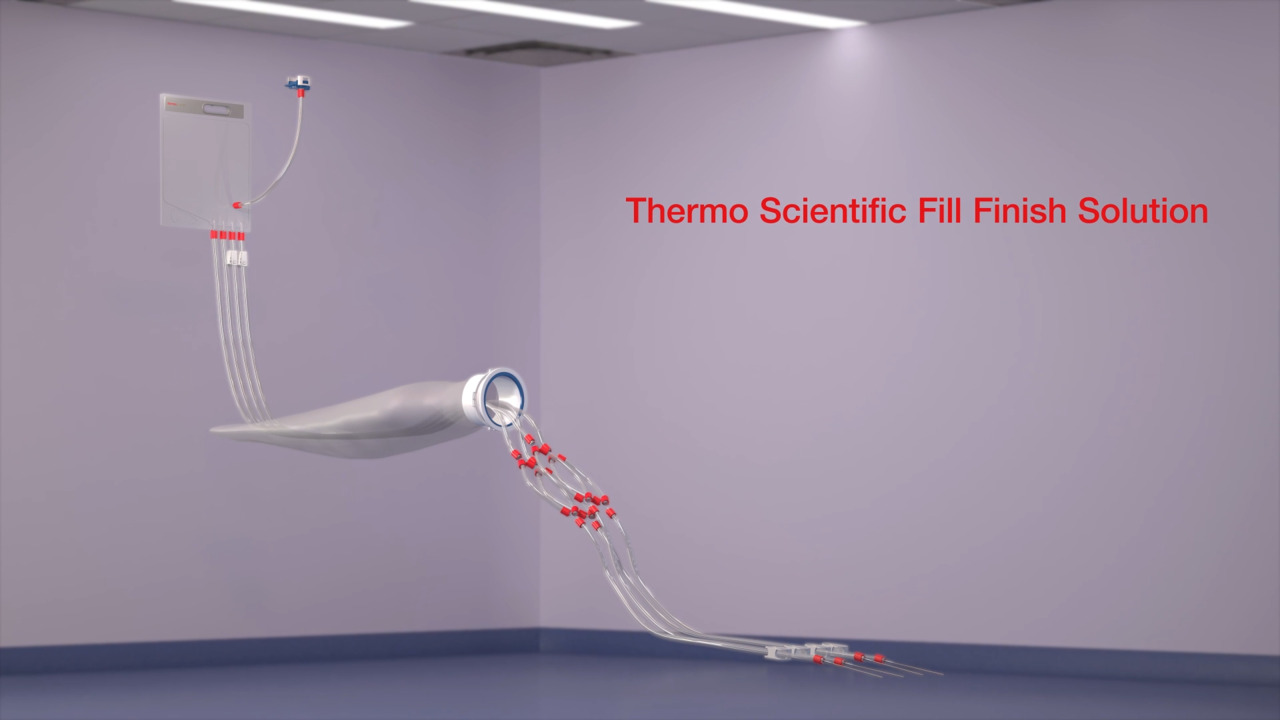
Fill Finish Solution Animated Video
2:16
Watch this overview video to learn how you can maximize drug product recovery with the Thermo Scientific Fill Finish Solution.













Lab Talk Episode 25: Maximizing Sustainability: Exploring Pipette Tip Recycling in the Lab
Watch as we explore sustainable practices in the laboratory by discussing the benefits and best practices associated with pipette tip recycling, aiming to reduce environmental impact and promote responsible laboratory waste management.
21:23

Utility of NGS in Lymphoid Malignancies and the Liverpool Lymphoid Network Panel experience
Learn how the Ion AmpliSeq Liverpool Lymphoid Network Panel can provide fast and comprehensive assessment of multiple lymphoid disorders in a single test.
17:37

Serum Smart: Where in the world is Grand Island, NY?
In this episode of Serum Smart, learn about Grand Island, NY and it's important history, and impact to cell culture research.
2:47

Avoid dish disasters with Nunc EasYDish cell culture dishes
Thermo Scientific Nunc EasYDish round cell culture dishes are designed to improve handling, stacking, and transporting of cell cultures in a lab.
1:00

LysoLight Deep Red antibody internalization
LysoLight Deep Red provides a powerful tool for visualizing the lysosomal delivery and catabolic degradation of antibodies and proteins.
0:22

How to optimize your parameters on the Neon NxT system
This video shows you the key steps to optimize your parameters on the Neon NxT Electroporation System. By understanding the importance of factors like pulse voltage, pulse width, pulse number, cell density, cell health,
6:21

Care & maintenance: Thermo Scientific™ SpeedVac™ vacuum concentrators
Learn how to perform routine care and maintenance for your Thermo Scientific SpeedVac Vacuum concentrator, so you can ensure years of functionality.
4:36


Centrifuge Room Virtual Experience
Tour the Thermo Scientific centrifuge room virtual experience.
1:10

ARL iSpark Plus OES Metal Analyzer – Taking speed and stability to the next level
The ARL iSpark Plus metal analyzer, the next generation of the trusted standard in spark optical emission spectrometry, streamlines your laboratory workflow by taking speed and stability to the next level.
3:07

The Thermo Scientific DynaChrom Single-Use Chromatography System
DynaChrom is a configurable system that alleviates operational burdens, streamlines workflows, and provides the tools necessary for a successful and hassle-free chromatography experience tailored to your unique requirements.
2:41

Fill Finish Solution Animated Video
Watch this overview video to learn how you can maximize drug product recovery with the Thermo Scientific Fill Finish Solution.
2:16
Popular Videos
-
Play video Aseptic Techniques: Cell Culture Basics
Aseptic Techniques: Cell Culture Basics
The handbook and videos provide an introduction to cell culture, with a focus on aseptic technique, microbial contamination, laboratory safety and cell culture experiments.
5:07
-
Play video Cell Culture: Cell Culture Basics
Cell Culture: Cell Culture Basics
In this video, we present the basic equipment used in cell culture and proper way to set-up a laboratory. Cell culture refers to the removal of cells from an animal or plant and their subsequent growth in a favorable artificial environment.
4:33
-
Play video What Makes a “Good” Bioprocess?
What Makes a “Good” Bioprocess?
The article introduces three main questions a manufacturer should ask themselves: 1) How do I simplify my process? 2) How do I ensure consistent performance? 3) How is upstream impacting downstream?
1:04
-
Play video Passaging Cells: Cell Culture Basics
Passaging Cells: Cell Culture Basics
This video provides an introduction to cell culture, with a focus on maintaining cell health throughout the processes of culturing, freezing, thawing and passaging cells. In this video, we focus on how to passage cells.
5:22
-
Play video Step-by-step guide to successful western blot analysis
Step-by-step guide to successful western blot analysis
Western Blot Analysis is the most widely used technique for protein detection. In this video, we will cover the 3 major steps in generating a western blot: Separate, Transfer, and Detect.
3:05
-
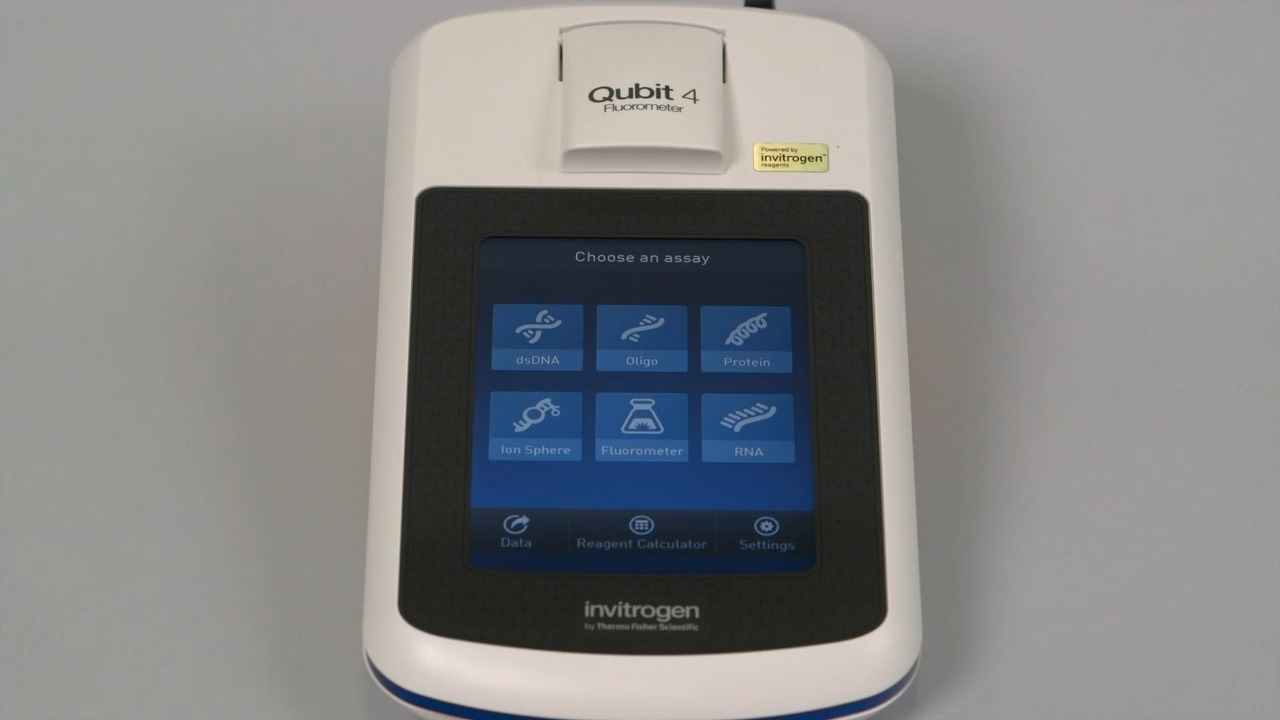
Play video Quantify DNA, RNA, or protein with the Qubit 4 Fluorometer
Quantify DNA, RNA, or protein with the Qubit 4 Fluorometer
Quickly quantitate the amount of DNA, RNA, or protein for proper preparation of the next step in your experiment. The quantitation process is simple, fast and even includes a built-in Reagent Calculator.
1:45
-
Play video How to separate proteins using an Invitrogen precast SDS-PAGE gel
How to separate proteins using an Invitrogen precast SDS-PAGE gel
Learn how to separate proteins by using a Invitrogen Bolt Bis-Tris Plus SDS-PAGE precast gel and Mini Gel Tank
5:24
-
Play video Invitrogen E-Gel Power Snap System Features
Invitrogen E-Gel Power Snap System Features
Learn about the E-Gel Power Snap System powerful features and how the integrated design combines the convenience of rapid, real-time nucleic acid analysis with high-resolution image capture to reduce workflow time and accelerate discovery.
1:51
-
Play video Thawing Cells: Cell Culture Basics
Thawing Cells: Cell Culture Basics
This video provides an introduction to cell culture, with a focus on maintaining cell health throughout the processes of culturing, freezing, thawing and passaging cells. In this video, we focus on how to thaw cells.
3:57
-
Play video Introduction to PCR
Introduction to PCR
PCR is a powerful technique in molecular biology. This video covers PCR overview, historical significance, and implications.
2:09
-
Play video How to stabilize RNA in fresh specimens
How to stabilize RNA in fresh specimens
Learn how to quickly and easily stabilize and protect RNA in fresh specimens. RNAlater Stabilization Solution eliminates the need to immediately process samples, freeze them in liquid nitrogen, or rush them to the freezer.
1:51
-
Play video How To Quickly Purify DNA with E-Gel CloneWell II Gels
How To Quickly Purify DNA with E-Gel CloneWell II Gels
Learn how to perform gel purification using the E-Gel Power Snap System and E-Gel CloneWell II gel. Load your sample, run the gel, then simply pipet out your purified DNA band and you’re done. No additional purification kits or steps are required.
2:21
What's New
-
Play video Lab Talk Episode 25: Maximizing Sustainability: Exploring Pipette Tip Recycling in the Lab
Lab Talk Episode 25: Maximizing Sustainability: Exploring Pipette Tip Recycling in the Lab
Watch as we explore sustainable practices in the laboratory by discussing the benefits and best practices associated with pipette tip recycling, aiming to reduce environmental impact and promote responsible laboratory waste management.
21:23
-
Play video Utility of NGS in Lymphoid Malignancies and the Liverpool Lymphoid Network Panel experience
Utility of NGS in Lymphoid Malignancies and the Liverpool Lymphoid Network Panel experience
Learn how the Ion AmpliSeq Liverpool Lymphoid Network Panel can provide fast and comprehensive assessment of multiple lymphoid disorders in a single test.
17:37
-
Play video Serum Smart: Where in the world is Grand Island, NY?
Serum Smart: Where in the world is Grand Island, NY?
In this episode of Serum Smart, learn about Grand Island, NY and it's important history, and impact to cell culture research.
2:47
-
Play video Avoid dish disasters with Nunc EasYDish cell culture dishes
Avoid dish disasters with Nunc EasYDish cell culture dishes
Thermo Scientific Nunc EasYDish round cell culture dishes are designed to improve handling, stacking, and transporting of cell cultures in a lab.
1:00
-
Play video LysoLight Deep Red antibody internalization
LysoLight Deep Red antibody internalization
LysoLight Deep Red provides a powerful tool for visualizing the lysosomal delivery and catabolic degradation of antibodies and proteins.
0:22
-
Play video How to optimize your parameters on the Neon NxT system
How to optimize your parameters on the Neon NxT system
This video shows you the key steps to optimize your parameters on the Neon NxT Electroporation System. By understanding the importance of factors like pulse voltage, pulse width, pulse number, cell density, cell health,
6:21
-
Play video Care & maintenance: Thermo Scientific™ SpeedVac™ vacuum concentrators
Care & maintenance: Thermo Scientific™ SpeedVac™ vacuum concentrators
Learn how to perform routine care and maintenance for your Thermo Scientific SpeedVac Vacuum concentrator, so you can ensure years of functionality.
4:36
-
Play video Genomics workflow 3D tour
Genomics workflow 3D tour
Preview the genomics workflow 3D tour.
0:50
-
Play video Centrifuge Room Virtual Experience
Centrifuge Room Virtual Experience
Tour the Thermo Scientific centrifuge room virtual experience.
1:10
-
Play video ARL iSpark Plus OES Metal Analyzer – Taking speed and stability to the next level
ARL iSpark Plus OES Metal Analyzer – Taking speed and stability to the next level
The ARL iSpark Plus metal analyzer, the next generation of the trusted standard in spark optical emission spectrometry, streamlines your laboratory workflow by taking speed and stability to the next level.
3:07
-
Play video The Thermo Scientific DynaChrom Single-Use Chromatography System
The Thermo Scientific DynaChrom Single-Use Chromatography System
DynaChrom is a configurable system that alleviates operational burdens, streamlines workflows, and provides the tools necessary for a successful and hassle-free chromatography experience tailored to your unique requirements.
2:41
-
Play video Fill Finish Solution Animated Video
Fill Finish Solution Animated Video
Watch this overview video to learn how you can maximize drug product recovery with the Thermo Scientific Fill Finish Solution.
2:16