Transitioning Lab Equipment from R&D work to GMP Compliance
35:29
Transitioning Laboratory Equipment from R&D work to GMP Compliance
Related Videos
In Cell & Gene Therapy Videos
-
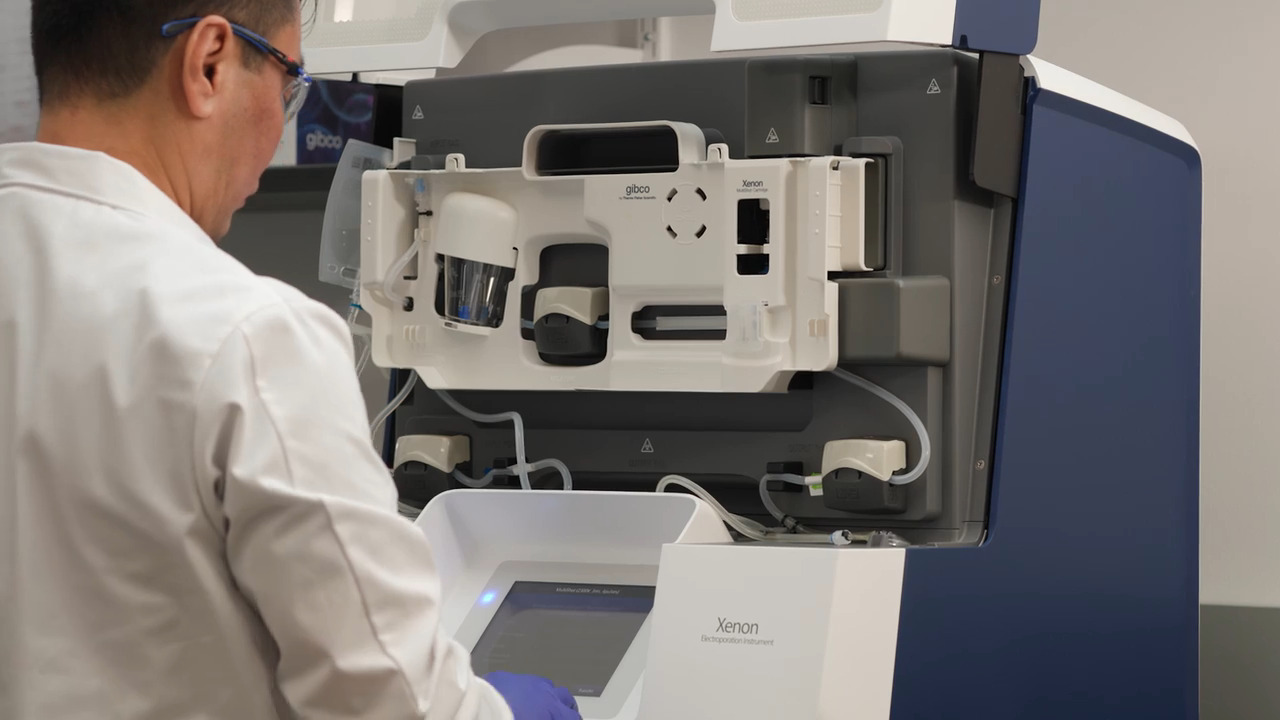
Play video CTS Xenon System - Meet The System
CTS Xenon System - Meet The System
Made for cell therapy process development and clinical manufacturing, the CTS Xenon System delivers high-performance electroporation in a closed system.
1:02
-
Play video Achieving high nonviral transfection performance for cell therapy processing
Achieving high nonviral transfection performance for cell therapy processing
CGTI webinar featuring Nektaria Andronikou, Thermo Fisher scientist, discussing the Gibco CTS Xenon Electroporation System for cell therapy.
53:58
-
Play video CTS Xenon System 30 Second Overview
CTS Xenon System 30 Second Overview
The Gibco CTS Xenon Electroporation System delivers high-performance nonviral transfection for cell therapy applications.
0:38
-
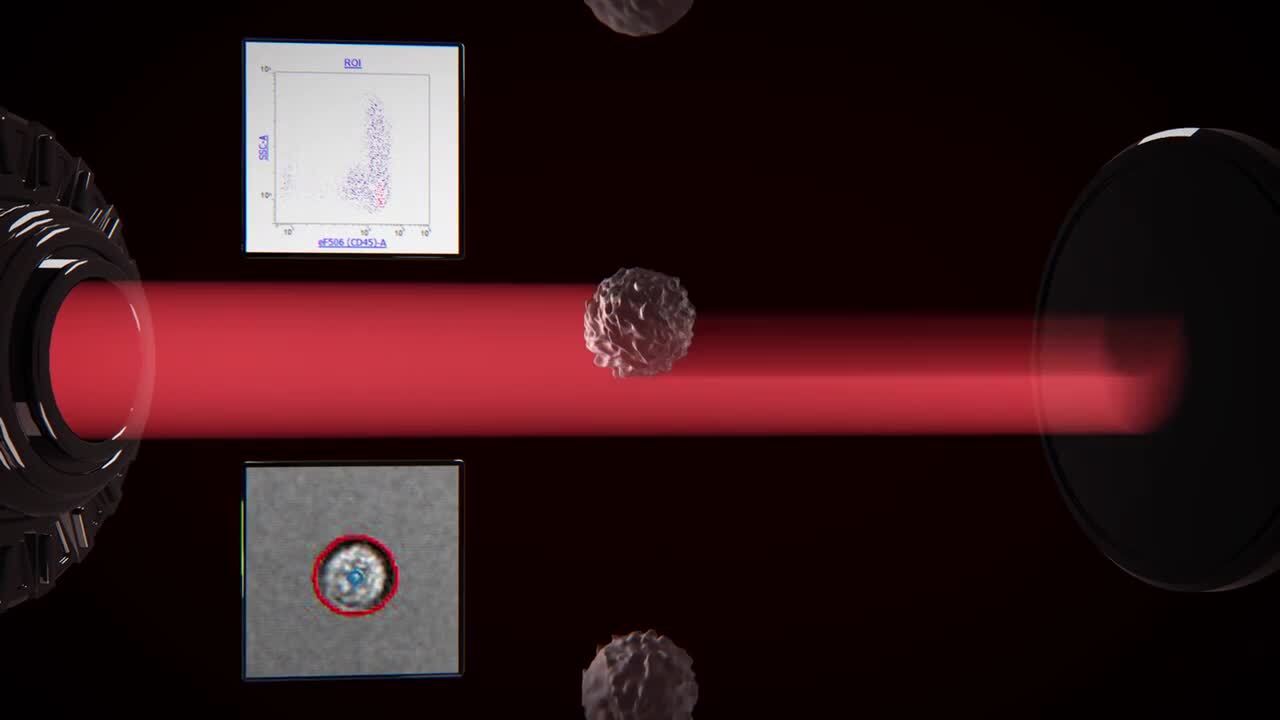
Play video Automated Image Analysis with the Attune CytPix flow cytometer
Automated Image Analysis with the Attune CytPix flow cytometer
The Invitrogen™ Attune™ CytPix™ Flow Cytometer enables image capture and processing with the new version of the Invitrogen™ Attune™Cytometric Software. You can now measure cell morphology parameters concurrently with flow cytometric parameters.
1:15
-
Play video CTS Xenon System 15 Second Overview
CTS Xenon System 15 Second Overview
The Gibco CTS Xenon Electroporation System delivers high-performance nonviral transfection for cell therapy applications.
0:26
-
Play video CTS Xenon System - How It Works
CTS Xenon System - How It Works
The Xenon System enables electroporation of up to 2.5 billion T cells in 25 mL for clinical cell therapy process development and manufacturing work.
1:43