Transitioning Lab Equipment from R&D work to GMP Compliance
35:29
Transitioning Laboratory Equipment from R&D work to GMP Compliance
Related Videos
In Cell & Gene Therapy Videos
-
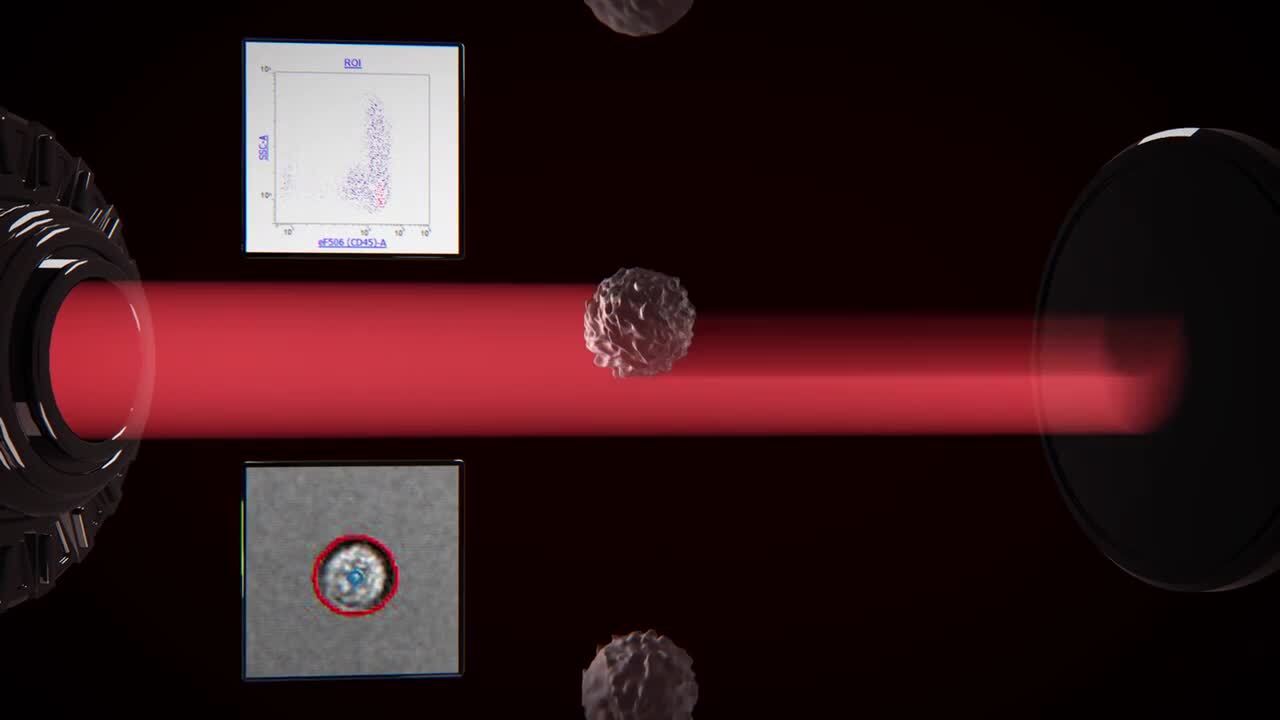
Play video Automated Image Analysis with the Attune CytPix flow cytometer
Automated Image Analysis with the Attune CytPix flow cytometer
The Invitrogen™ Attune™ CytPix™ Flow Cytometer enables image capture and processing with the new version of the Invitrogen™ Attune™Cytometric Software. You can now measure cell morphology parameters concurrently with flow cytometric parameters.
1:15
-
Play video CTS Xenon System 30 Second Overview
CTS Xenon System 30 Second Overview
The Gibco CTS Xenon Electroporation System delivers high-performance nonviral transfection for cell therapy applications.
0:38
-
Play video CTS Xenon System - SingleShot Chamber
CTS Xenon System - SingleShot Chamber
The Xenon SingleShot chamber enables rapid electroporation of up to 1 mL of cells, and was designed for cell therapy process development.
1:23
-
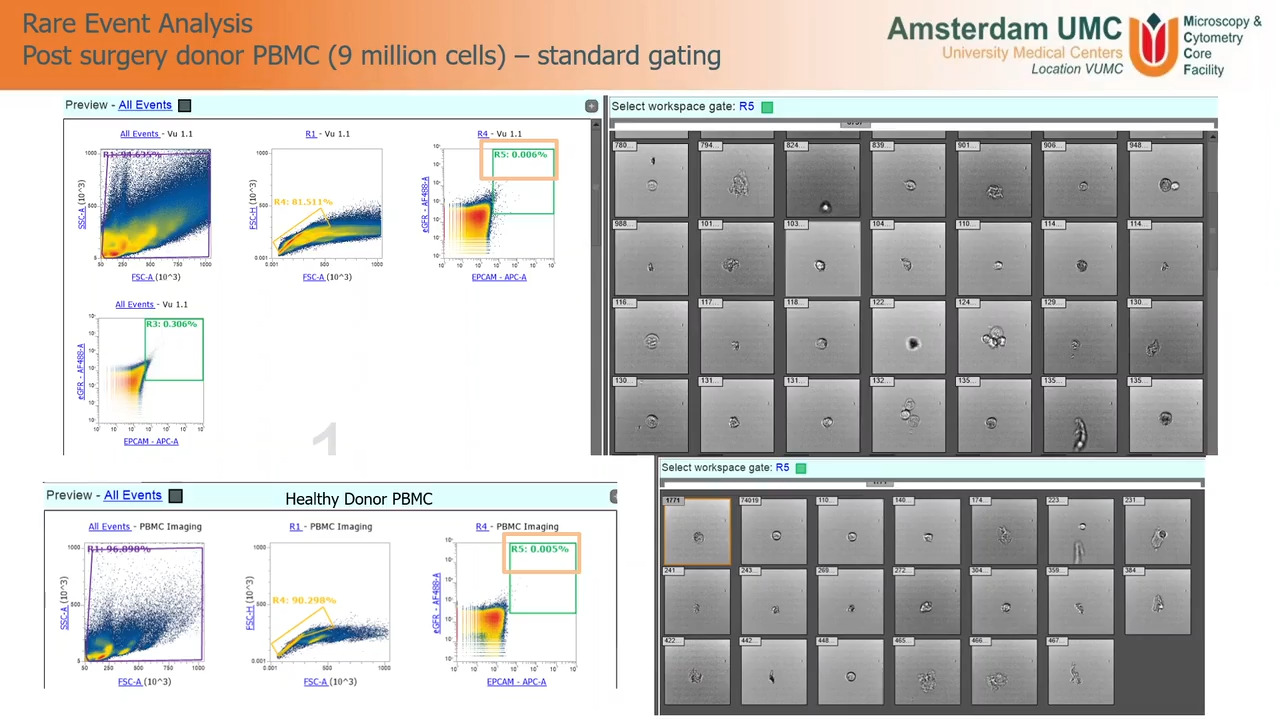
Play video Brightfield imaging-enabled flow cytometry with the Attune CytPix
Brightfield imaging-enabled flow cytometry with the Attune CytPix
Learn how the imaging-enhanced Attune CytPix flow cytometer is being used by an academic core facility to help users optimize their gating strategy.
33:36
-
Play video CTS Xenon System - MultiShot Cartridge
CTS Xenon System - MultiShot Cartridge
The Xenon MultiShot cartridge enables consistent electroporation of up to 25 mL in under 25 minutes in a closed, GMP-compliant system.
2:25
-
Play video CTS Xenon System 15 Second Overview
CTS Xenon System 15 Second Overview
The Gibco CTS Xenon Electroporation System delivers high-performance nonviral transfection for cell therapy applications.
0:26