-
Play video The Upstream Advantage: Unlocking bioprocessing potential
The Upstream Advantage: Unlocking bioprocessing potential
Hear from Thermo Fisher Scientific colleagues and learn about their perspectives on the future of bioprocessing.
3:26
-
Play video Gibco Efficient Pro Feed 3 Protein Production Workflow
Gibco Efficient Pro Feed 3 Protein Production Workflow
Boost CHO-K1 GS titers by up to 146% with Efficient-Pro Feed 3 and Feed Enhancer—a two-part, chemically defined, animal-origin-free system designed with advanced metabolomics to enhance productivity and maintain critical product quality.
1:41
-
Play video How to operate a Thermo Scientific DynaDrive 5L Single-Use Bioreactor
How to operate a Thermo Scientific DynaDrive 5L Single-Use Bioreactor
Watch a walkthrough of how to properly operate a Thermo Scientific DynaDrive 5L Single-Use Bioreactor.
8:28
-
Play video The Upstream Advantage: Unlocking bioprocessing potential
The Upstream Advantage: Unlocking bioprocessing potential
Listen to various Thermo Fisher Scientific colleagues share their insights on the future of bioprocessing.
8:00
-
Play video Affinity Chromatography: Innovative Approach for Enhanced Yield and Purity in Plasma-derived IgG
Affinity Chromatography: Innovative Approach for Enhanced Yield and Purity in Plasma-derived IgG
A robust IVIG production method using direct adsorption & affinity chromatography enhances IgG yield, purity, and subclass retention, reduces impurities, enables resin reuse for cost savings & removes ethanol precipitation for optimized processing.
31:23
-
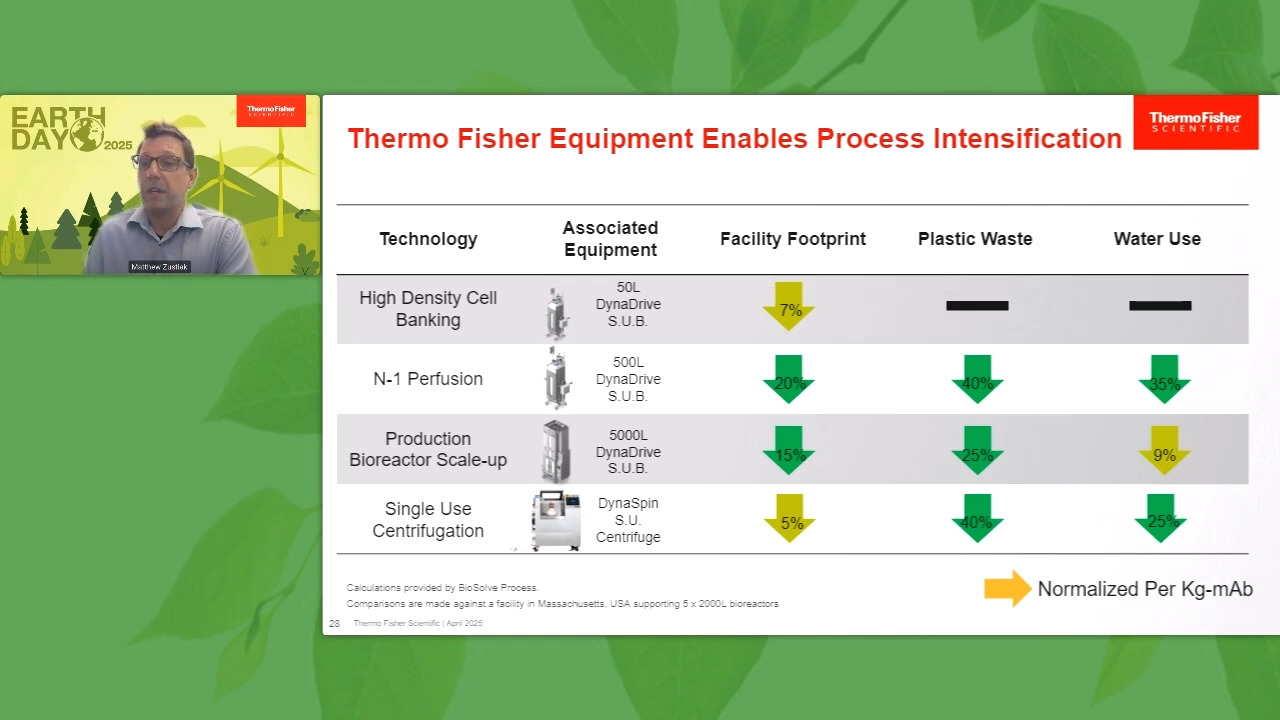
Play video Sustainable Manufacturing through Efficient Bioproduction Unit Operations
Sustainable Manufacturing through Efficient Bioproduction Unit Operations
Ready to discover how to make bioprocessing greener and more cost-effective? Learn about key environmental and economic impacts, the urgency of managing plastic waste, and a three-pronged strategy to address this growing industry challenge.
34:24
-
Play video Navigating global regulatory landscapes in an evolving biopharma industry
Navigating global regulatory landscapes in an evolving biopharma industry
Join our webinar for expert insights, engaging discussions, and interactive Q&A sessions. Discover the latest trends, expand your knowledge, and connect with professionals in your field. Don’t miss this chance to enhance your skills and stay ahead!
27:52
-
Play video E-Gel Power Snap Plus Cinematic
E-Gel Power Snap Plus Cinematic
The Invitrogen E-Gel Power Snap Plus Electrophoresis System is a compact, precast agarose gel running and imaging station.
0:39
-
Play video CaptureSelect Affinity Antibody Resins Cinematic
CaptureSelect Affinity Antibody Resins Cinematic
CaptureSelect® products possess a combination of unique properties, including selectivity, affinity, and stability.
1:01
-
Play video Single-Use Assembly Configurator Tutorial Video
Single-Use Assembly Configurator Tutorial Video
Our Single-Use Assembly Configurator streamlines your design process and saves time. The online tool is free to access, requires no login, and features precise design capabilities with 3D renderings to help visualize and meet your assembly needs.
6:20
-
Play video Introducing the Single-Use Assembly Configurator
Introducing the Single-Use Assembly Configurator
An easy-to-use selection tool that streamlines the design process and saves you valuable time.
0:33
-
Play video Introducing the Single-Use DynaDrive SUB 5L Bioreactor
Introducing the Single-Use DynaDrive SUB 5L Bioreactor
Learn more about the latest Thermo Scientific DynaDrive Single-Use Bioreactor—a benchtop model that offers an exceptional level of performance.
0:56
- Load More
Shopping Tool
eSolutions
Thermo Fisher Scientific
Note: You clicked on an external link, which has been disabled in order to keep your shopping session open.
Ok
Share this video
Embed
Size: x pixels