-
Play video Fast, Flexible, and Spectrally Brilliant: Invitrogen Attune Xenith Flow Cytometer
Fast, Flexible, and Spectrally Brilliant: Invitrogen Attune Xenith Flow Cytometer
Fast, flexible, and spectrally brilliant—simplify high-speed analysis with the Invitrogen Attune Xenith Flow Cytometer. Visit thermofisher.com/attunexenith.
1:00
-
Play video Absolute Gene-ius: S2 E10 Automating accuracy – an insider’s view
Absolute Gene-ius: S2 E10 Automating accuracy – an insider’s view
Automation is key to enabling high-throughput analysis for any analytical method, and automation of digital PCR (dPCR) is now a reality. Join us for this conversation with Dr. Clarance Lee, Senior Product Manger
31:47
-
Play video Absolute Gene-ius: S2 E9 Helping democratize access and use of mRNA technology
Absolute Gene-ius: S2 E9 Helping democratize access and use of mRNA technology
Learn how our guest is working to make high-purity mRNA available to more researchers and drug developers to help realize even more potential from mRNA.
32:49
-
Play video Absolute Gene-ius: S2 E6 What’s your vector, Victor?
Absolute Gene-ius: S2 E6 What’s your vector, Victor?
Curious about cell and gene therapy and the viral vectors used in these applications? Join us for a conversation to shed light on these topics.
35:28
-
Play video Absolute Gene-ius: S2 E2 CAR-T loads of immunology insights
Absolute Gene-ius: S2 E2 CAR-T loads of immunology insights
This episode, which focuses on CAR-T cell therapy. Join us to meet Raquel Munoz and learn about what she thinks will be the future of cancer research
31:59
-
Play video Absolute Gene-ius S2 E1
Absolute Gene-ius S2 E1
A couple of reproductive biology experts
0:28
-
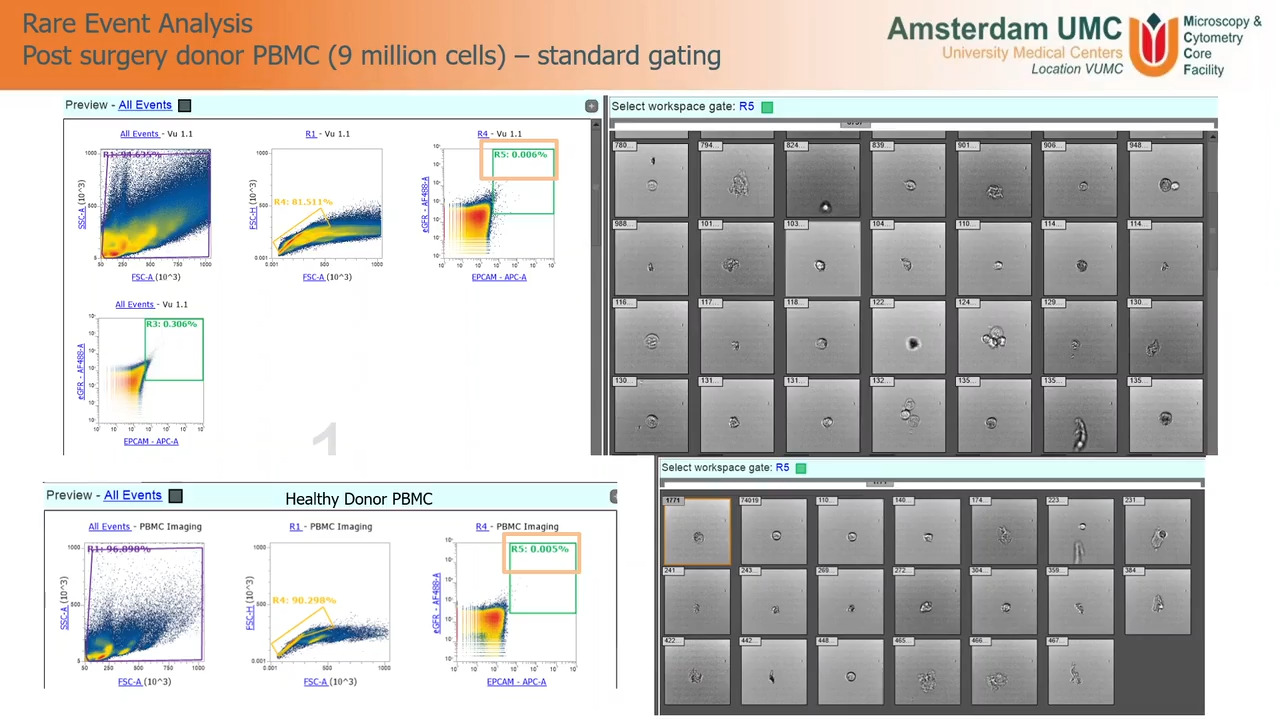
Play video Brightfield imaging-enabled flow cytometry with the Attune CytPix
Brightfield imaging-enabled flow cytometry with the Attune CytPix
Learn how the imaging-enhanced Attune CytPix flow cytometer is being used by an academic core facility to help users optimize their gating strategy.
33:36
-
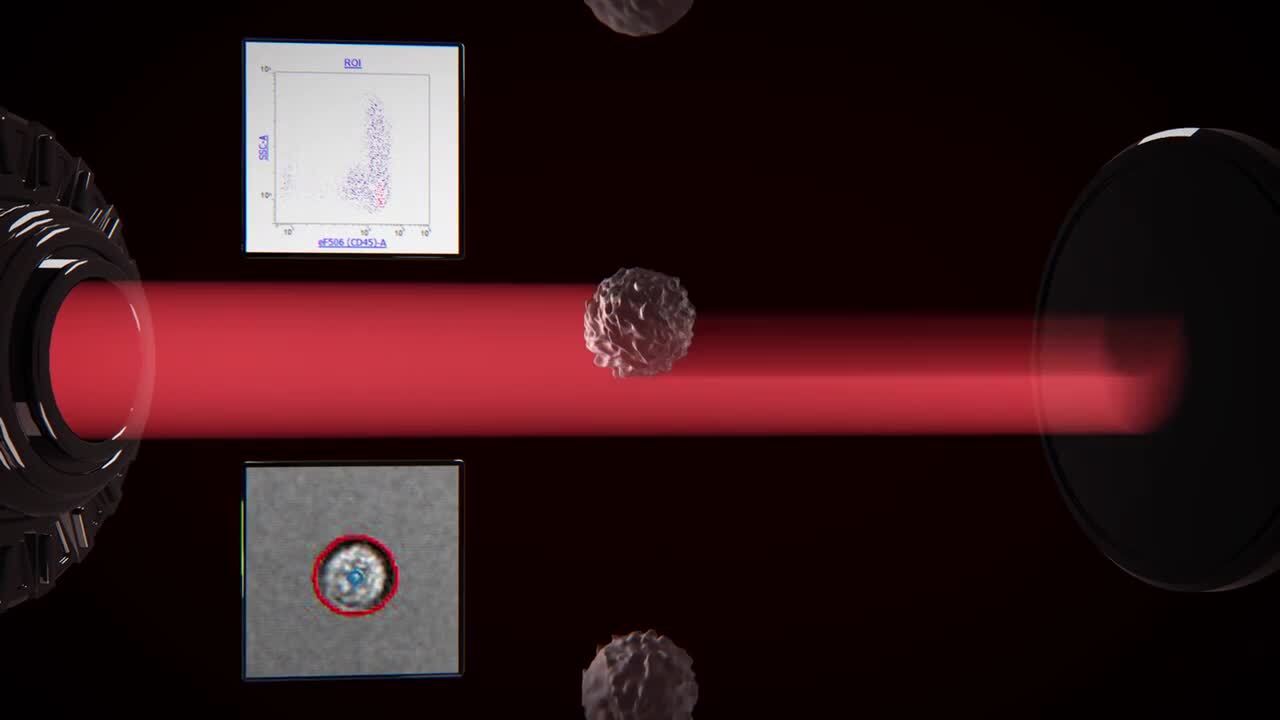
Play video Automated Image Analysis with the Attune CytPix flow cytometer
Automated Image Analysis with the Attune CytPix flow cytometer
The Invitrogen™ Attune™ CytPix™ Flow Cytometer enables image capture and processing with the new version of the Invitrogen™ Attune™Cytometric Software. You can now measure cell morphology parameters concurrently with flow cytometric parameters.
1:15
-
Play video Achieving high nonviral transfection performance for cell therapy processing
Achieving high nonviral transfection performance for cell therapy processing
CGTI webinar featuring Nektaria Andronikou, Thermo Fisher scientist, discussing the Gibco CTS Xenon Electroporation System for cell therapy.
53:58
-
Play video Serum-free Expansion of NK Cells with K562- Based Feeder Systems for Clinical Applications
Serum-free Expansion of NK Cells with K562- Based Feeder Systems for Clinical Applications
Webinar on Serum-free Expansion of NK Cells with K562- Based Feeder Systems for Clinical Applications featuring Dean Lee, MD, Nationwide Children's Hospital.
1:01:44
-
Play video CTS Xenon System - How It Works
CTS Xenon System - How It Works
The Xenon System enables electroporation of up to 2.5 billion T cells in 25 mL for clinical cell therapy process development and manufacturing work.
1:43
-
Play video CTS Xenon System 15 Second Overview
CTS Xenon System 15 Second Overview
The Gibco CTS Xenon Electroporation System delivers high-performance nonviral transfection for cell therapy applications.
0:26
- Load More
Shopping Tool
eSolutions
Thermo Fisher Scientific
Note: You clicked on an external link, which has been disabled in order to keep your shopping session open.
Ok
Share this video
Embed
Size: x pixels