Gibco CTS DynaMag Magnet: Bead Removal and Cell Harvesting
2:39
An overview of the Gibco CTS DynaMag Magnet and how to use it for harvesting T Cells using Gibco CTS Dynabeads CD3/CD28 magnetic beads.
Related Videos
In Bioprocessing Videos
-
Play video CTS DynaMag Magnet: Cell Isolation | Gibco
CTS DynaMag Magnet: Cell Isolation | Gibco
A step-by-step guide for using the Gibco CTS DynaMag Magnet for the isolation of T cells to be used in cell therapy.
4:15
-
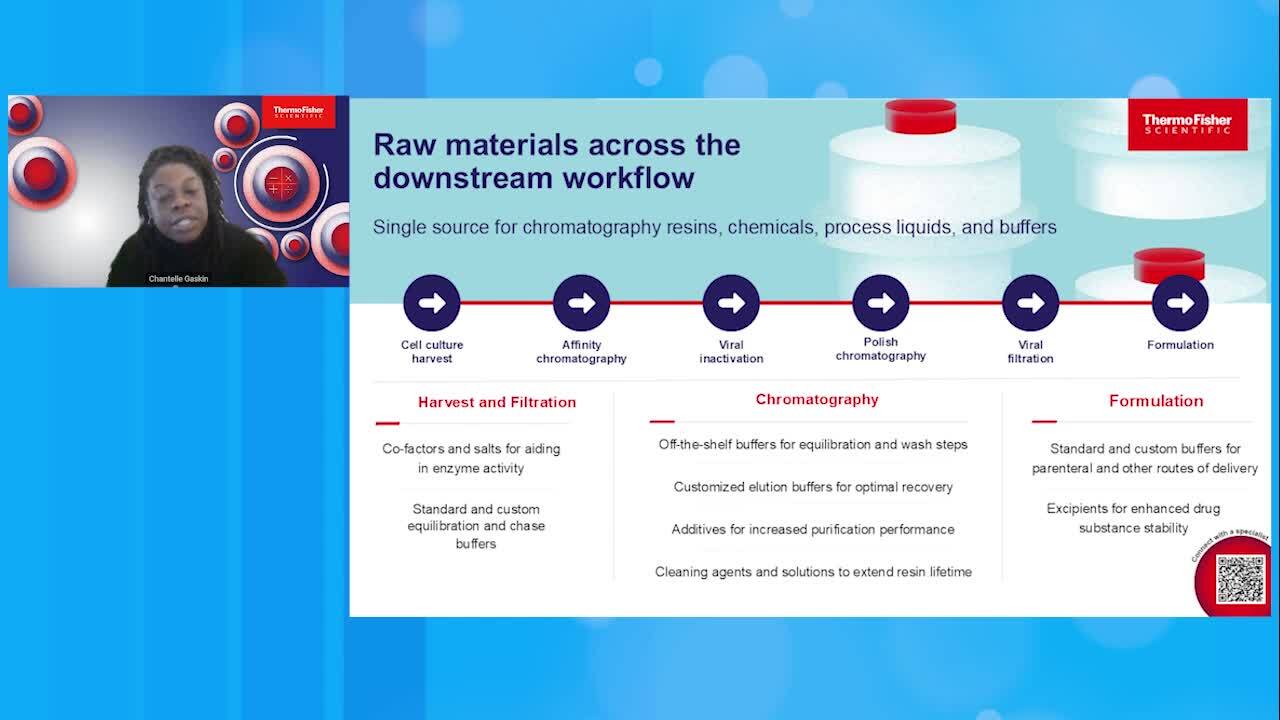
Play video Thermo Fisher Scientific Bioprocessing Downstream Solutions and Calculator
Thermo Fisher Scientific Bioprocessing Downstream Solutions and Calculator
Discover a scalable portfolio of downstream bioprocessing solutions--chromatography resins, chemicals, process liquids, buffers and supply chain services--that can help you maximize productivity while reducing costs and time to market.
16:47
-
Play video Dry Powder Media Manufacturing Grand Island Expansion
Dry Powder Media Manufacturing Grand Island Expansion
Take a video tour of our newly expanded dry powder media manufacturing facility in Grand Island, New York. The expansion has added more than 45,000 square feet of Animal Origin Free (AOF) manufacturing space to help meet increasing global demand.
1:40
-
Play video Thermo Scientific Bioproduction Sustain Program
Thermo Scientific Bioproduction Sustain Program
The first of its kind Thermo Scientific™ Bioproduction Sustain™ Program is an opportunity to reduce annual carbon emissions when purchasing the same trusted single-use bioprocessing containers and cell factories. Learn how: thermofisher.com/sustain
0:46
-
Play video Enabling Assurance of Supply Through Evolving Bioprocessing Needs
Enabling Assurance of Supply Through Evolving Bioprocessing Needs
This featured event at the Gibco™ cell culture media 60th-anniversary in Grand Island, New York spotlights proactive strategies used by Thermo Fisher Scientific to decrease risk, ensure quality and meet customers’ raw material needs worldwide.
15:16
-
Play video A Next-Gen Medium and Feeds System to Improve Productivity in CHO Cell Lines
A Next-Gen Medium and Feeds System to Improve Productivity in CHO Cell Lines
Learn how to more rapidly optimize upstream processes and seamlessly scale up with the Gibco™ Efficient-Pro™ system-- an enriched medium and feeds to support performance improvements in CHO-K1, CHO-K1 GS, CHO-S, and DG44 cells.
33:00