3K and 5K L DynaDrive systems preparing the tank
2:28
This video goes through the step-by-step process of unboxing and loading the BPC for the 5K DynaDrive Bioreactor.
Related Videos
In Bioprocessing Videos
-
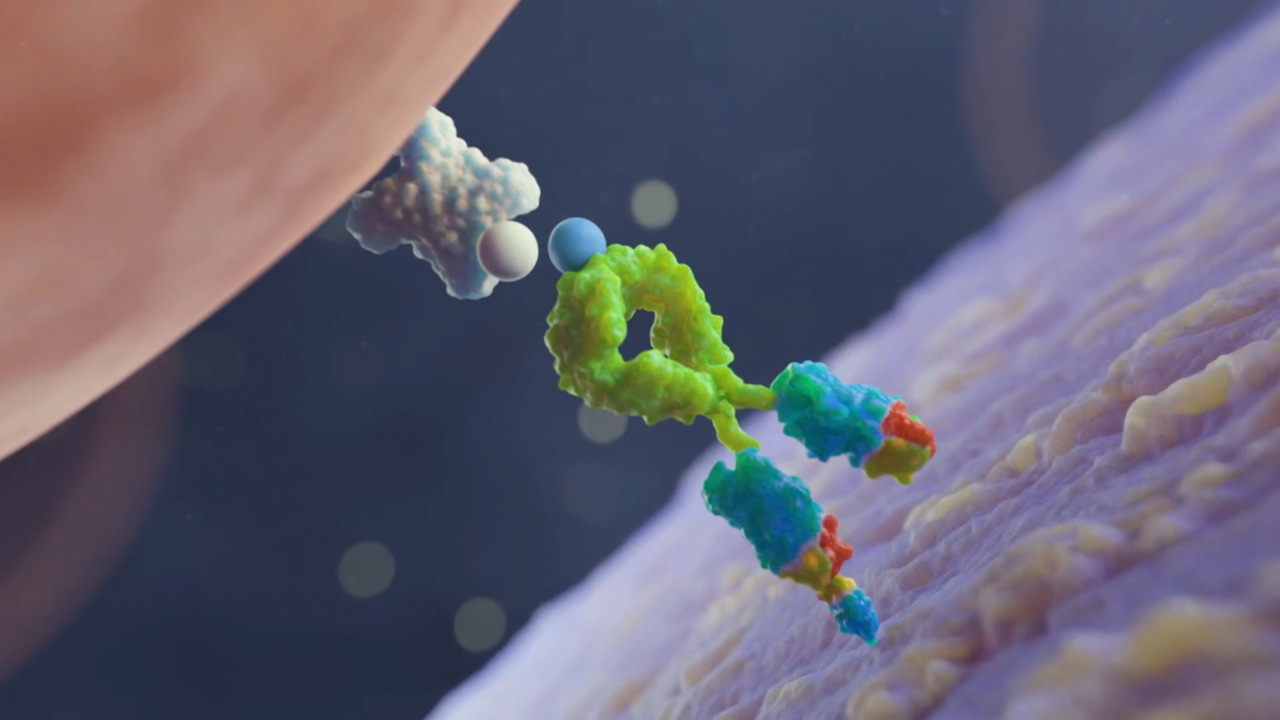
Play video Gibco CTS Detachable Dynabeads Overview
Gibco CTS Detachable Dynabeads Overview
The Gibco CTS Detachable Dynabeads are a breakthrough offering and are the first to have an active release mechanism for clinical trial and commercial manufacturing use.
1:22
-
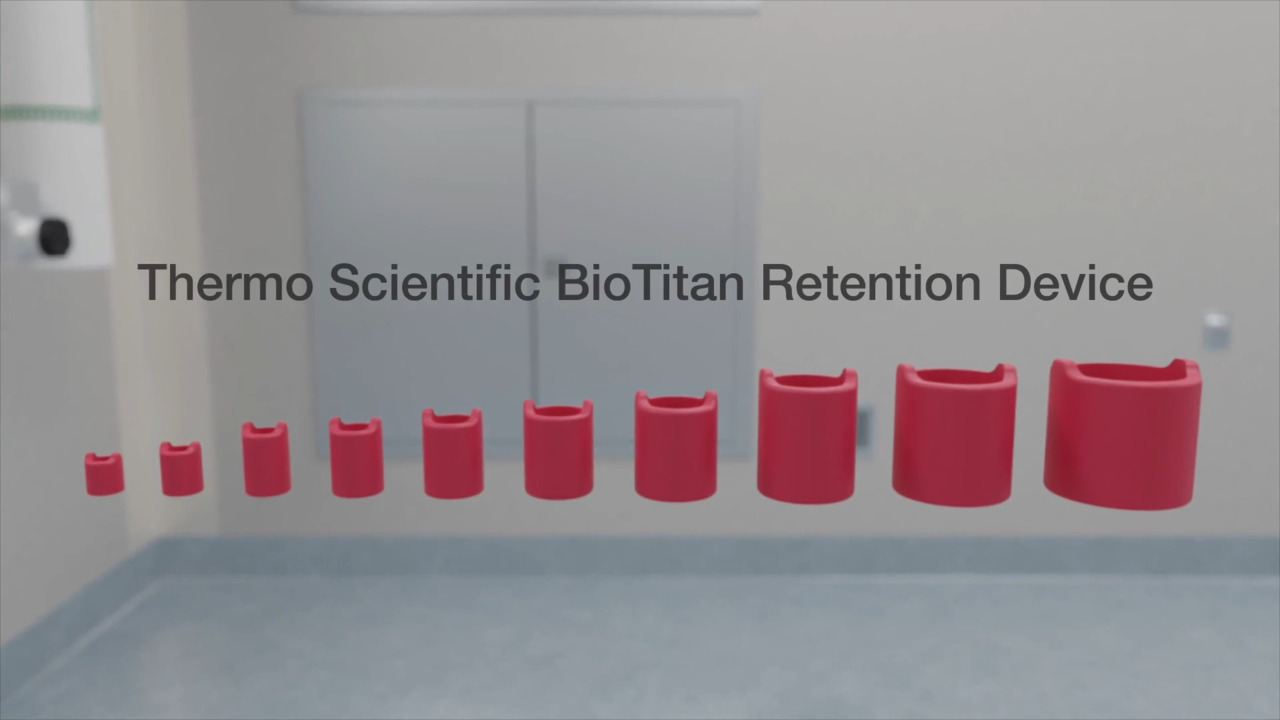
Play video Thermo Scientific BioTitan Retention Device cinematic
Thermo Scientific BioTitan Retention Device cinematic
Overview of the Thermo Scientific BioTitan Retention Device, featured on single-use products such as Labtainer Pro BioProcessing Container and compatible with Thermo Scientific equipment like the Thermo Scientific DynaDrive Single-Use Bioreactor.
1:53
-
Play video Mitigate Bioprocessing Limitations with a More Efficient and Sustainable Harvest Solution
Mitigate Bioprocessing Limitations with a More Efficient and Sustainable Harvest Solution
Subject matter experts will introduce the Thermo Scientific™ DynaSpin™ Single-Use Centrifuge. This conversation will be focused on the research and development of this harvesting solution and how this innovation can solve customer needs.
39:48
-
Play video How to use 5 and 10L BPCs of the Gibco CTS OpTmizer family of serum free media
How to use 5 and 10L BPCs of the Gibco CTS OpTmizer family of serum free media
Gibco™ CTS™ OpTmizer™ T Cell Expansion SFM, no phenol red, and Gibco™ CTS™ OpTmizer™ Pro SFM are now available in 5 L and 10 L volumes in Thermo Scientific™ BioProcess Containers.
4:17
-
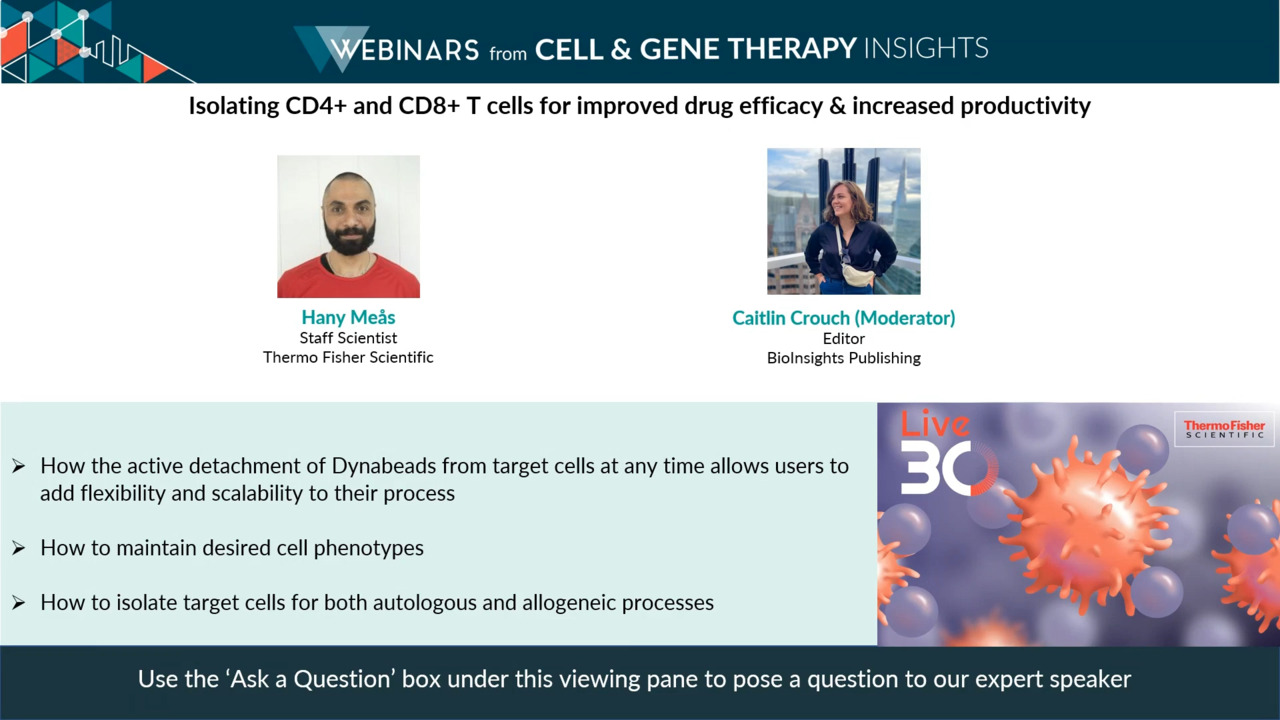
Play video Isolating CD4 / CD8 T cells for improved drug efficacy & productivity
Isolating CD4 / CD8 T cells for improved drug efficacy & productivity
Learn how the active detachment of dynabeads from target cells at any time allows users to add flexibility and scalability to their process.
32:04
-
Play video Knowledge Culture: Downstream innovation
Knowledge Culture: Downstream innovation
In this workshop, you’ll learn about various solutions, from cell isolation to cell expansion, that can support autologous and allogeneic cell therapy process needs, digital automation, and mycoplasma testing.
23:37