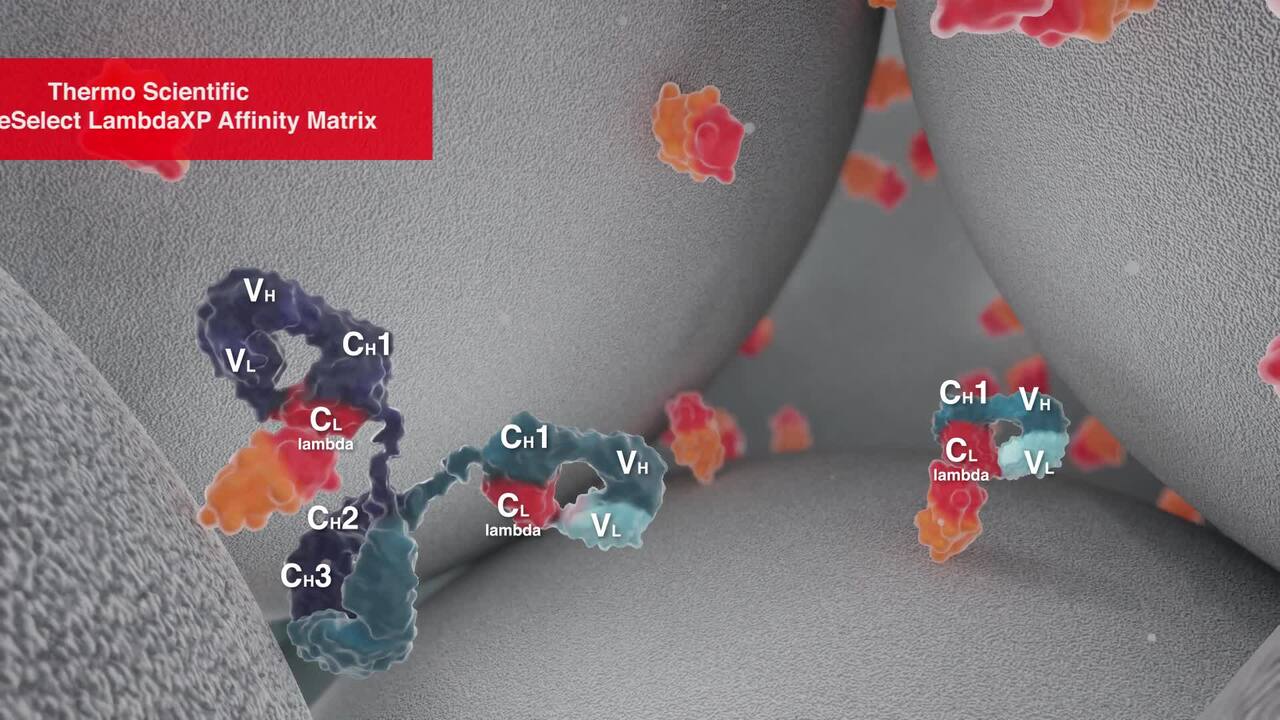
Quality Roundtable: Raw Material Planning for Tech Transfer and Scaling Biologics
40:40
Transitioning biopharmaceuticals from research to clinical and commercial manufacturing requires strategic planning as well as careful considerations for raw materials, process efficiency, regulatory compliance, and quality systems—and sometimes a trusted partner. Watch this roundtable to hear biopharmaceutical professionals share their insights to help you navigate important quality and planning decisions for the progression of your biologic. The panel answers questions about: -Overcoming challenges for tech transfer & scaling -Understanding vendor certificates of analysis -Raw material grades, documentation, selection & logistics -Preparation for process performance qualifications (PPQ) -Regulatory expectations, requirements & filings -Supplier auditing & qualification -Inventory & supply chain management