WHITE PAPER: Manufacturing pluripotent stem cell therapeutics
2:00
Description
Related Videos
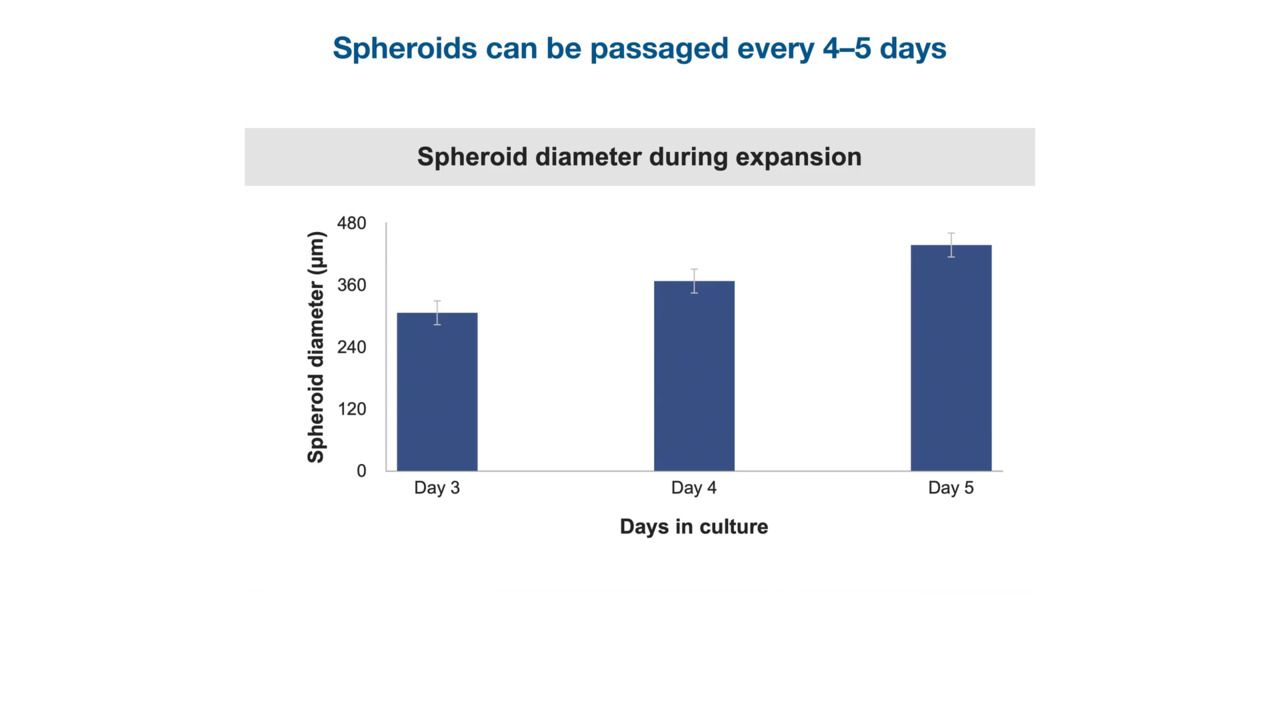
During the process of developing a cell therapeutic, it’s essential to plan at least a decade ahead and consider when regulatory approval will be pursued. This short video previews a white paper that discusses various aspects of pluripotent stem cell (PSC) therapy development, including cell therapy regulatory considerations, principles of cGMP, establishment of GMP cell banks, and more.
View More
View Less